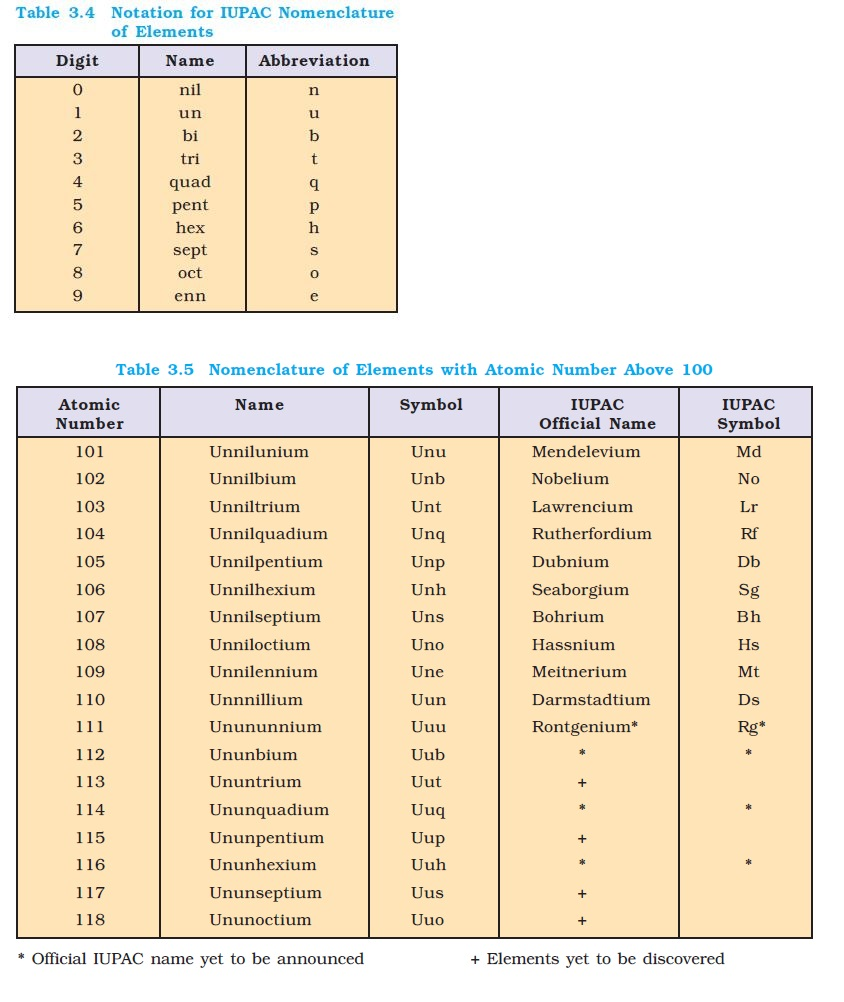
Modern Periodic Law and the Present Form of the Periodic Table :
`=>` In `1913`, the English physicist, Henry Moseley observed regularities in the characteristic `X`-ray spectra of the elements.
● A plot of `sqrtν` (where `ν` is frequency of `X`-rays emitted) against atomic number (`Z`) gave a straight line and not the plot of `sqrtν` vs atomic mass.
● He thereby showed that the atomic number is a more fundamental property of an element than its atomic mass.
● Mendeleev’s Periodic Law was, therefore, accordingly modified. This is known as the Modern Periodic Law and can be stated as :
`text(The physical and chemical properties of the elements are periodic functions of their atomic numbers)`.
`=>` The Periodic Law revealed important analogies among the 94 naturally occurring elements (neptunium and plutonium like actinium and protoactinium are also found in pitch blende – an ore of uranium).
`=>` It stimulated renewed interest in Inorganic Chemistry and has carried into the present with the creation of artificially produced short-lived elements.
`=>` The atomic number is equal to the nuclear charge (i.e., number of protons) or the number of electrons in a neutral atom.
● It is then easy to visualize the significance of quantum numbers and electronic configurations in periodicity of elements.
● In fact, it is now recognized that the Periodic Law is essentially the consequence of the periodic variation in electronic configurations, which indeed determine the physical and chemical properties of elements and their compounds.
`=>` Numerous forms of Periodic Table have been devised from time to time.
● Some forms emphasise chemical reactions and valence, whereas others stress the electronic configuration of elements.
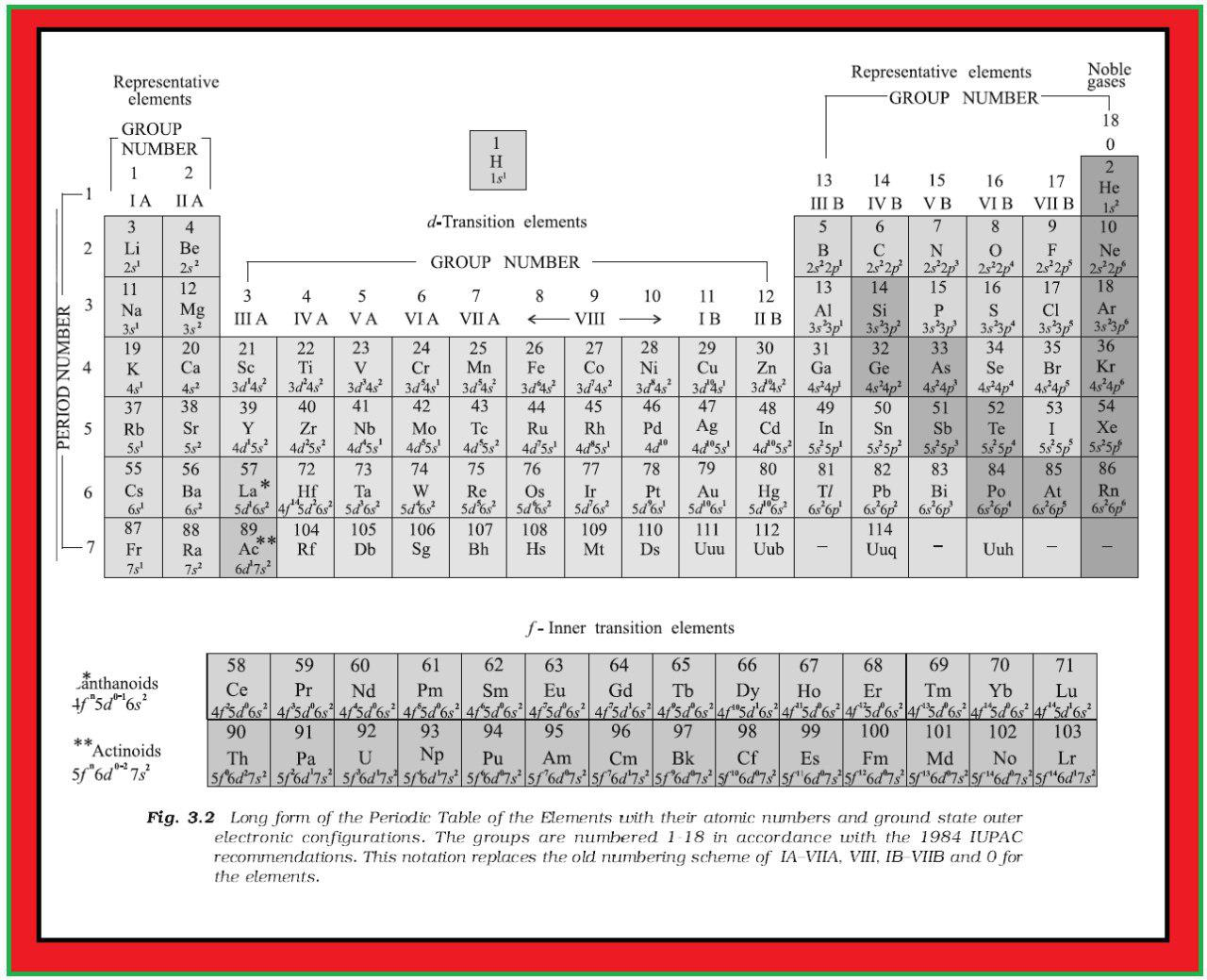
● A modern version, the so-called “long form” of the Periodic Table of the elements (Fig. 3.2), is the most convenient and widely used.
`text(Periods and Groups :)` The horizontal rows (which Mendeleev called series) are called periods and the vertical columns, groups.
`text(Groups or Families :)` Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups or families.
`=>` According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 replacing the older notation of groups IA … VIIA, VIII, IB … VIIB and 0.
`=>` There are altogether seven periods.
● The period number corresponds to the highest principal quantum number (`n`) of the elements in the period.
● The first period contains `2` elements.
● The subsequent periods consists of `8`, `8`, `18`, `18` and `32` elements, respectively.
● The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of `32` elements.
● In this form of the Periodic Table, `14` elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom.
● A plot of `sqrtν` (where `ν` is frequency of `X`-rays emitted) against atomic number (`Z`) gave a straight line and not the plot of `sqrtν` vs atomic mass.
● He thereby showed that the atomic number is a more fundamental property of an element than its atomic mass.
● Mendeleev’s Periodic Law was, therefore, accordingly modified. This is known as the Modern Periodic Law and can be stated as :
`text(The physical and chemical properties of the elements are periodic functions of their atomic numbers)`.
`=>` The Periodic Law revealed important analogies among the 94 naturally occurring elements (neptunium and plutonium like actinium and protoactinium are also found in pitch blende – an ore of uranium).
`=>` It stimulated renewed interest in Inorganic Chemistry and has carried into the present with the creation of artificially produced short-lived elements.
`=>` The atomic number is equal to the nuclear charge (i.e., number of protons) or the number of electrons in a neutral atom.
● It is then easy to visualize the significance of quantum numbers and electronic configurations in periodicity of elements.
● In fact, it is now recognized that the Periodic Law is essentially the consequence of the periodic variation in electronic configurations, which indeed determine the physical and chemical properties of elements and their compounds.
`=>` Numerous forms of Periodic Table have been devised from time to time.
● Some forms emphasise chemical reactions and valence, whereas others stress the electronic configuration of elements.
● A modern version, the so-called “long form” of the Periodic Table of the elements (Fig. 3.2), is the most convenient and widely used.
`text(Periods and Groups :)` The horizontal rows (which Mendeleev called series) are called periods and the vertical columns, groups.
`text(Groups or Families :)` Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups or families.
`=>` According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 replacing the older notation of groups IA … VIIA, VIII, IB … VIIB and 0.
`=>` There are altogether seven periods.
● The period number corresponds to the highest principal quantum number (`n`) of the elements in the period.
● The first period contains `2` elements.
● The subsequent periods consists of `8`, `8`, `18`, `18` and `32` elements, respectively.
● The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of `32` elements.
● In this form of the Periodic Table, `14` elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom.